Timeline: How Contaminated Syringes Got From Factory to Patients
U.S. Food and Drug Administration inspectors found problems at the AM2PAT factory in North Carolina but didn’t act in time to prevent a lot of 74,000 syringes from getting to patients in at least five states. On Dec. 20, 2007, the FDA recalled the syringes, citing bacterial contamination “that could present a serious adverse health consequence that could lead to life-threatening injuries and/or death.” At least 4 deaths and more than 160 infections were linked to the syringes.
Click on a thumbnail image to scroll to that event. Return to main story: Tainted Syringes Slipped Past FDA's Watch.
 |  |  |  |  |  |  |  |  |  |  |  |
| FDA/AM2PAT Actions | Victims | |
|---|---|---|
|
Aug. 4, 2003
Illinois businessman Dushyant Patel applies to the FDA to market syringes pre-filled with heparin. Patel lied on the application about completing "accelerated stability studies" of the syringes, according to an indictment against him.
Dec. 23, 2003
Apparently unaware of the problem, the FDA approves AM2PAT's application to manufacture syringes.
|
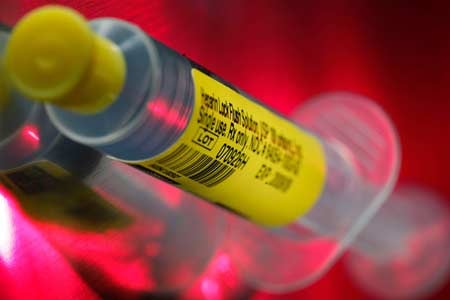
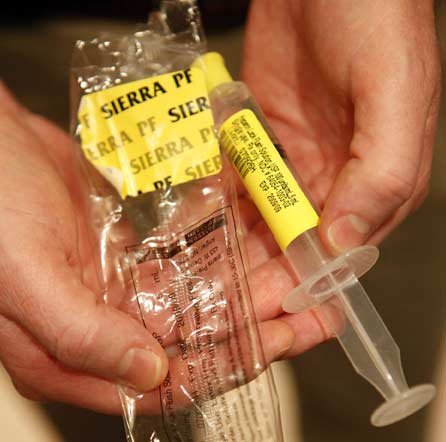
A pre-filled Heparin syringe made by AM2PAT under the brand Sierra Pre-Filled. The syringe shown came from the same lot of syringes linked to deaths and infections. Chris Walker/Chicago Tribune, May 11, 2009 
Eastern District of North Carolina grand jury indictment naming AM2PAT and Dushyant Patel, Feb. 19, 2009 |
|
|
March 2005
A pharmacist reports "copious amounts" of orange sediment in one AM2PAT syringe and a hair in another. In May, the company receives a complaint about brown material in a syringe but does not investigate the claim thoroughly or report it to the FDA, an FDA inspection report says.
|

Consumer Complaint/Injury Report to the FDA, Mar. 29, 2005 |
|
|
June 6, 2005
An FDA inspector spends six days at AM2PAT's Raleigh facility investigating reports of sediment and hair in the syringes. She notes that the company has minimal policies to assure that syringes are sterile and that they "constantly" fall off an inspection line on to the floor. The inspector finds glue traps loaded with insects and an employee chewing gum while filling syringes. She watches workers standing in front of a fast-moving conveyor belt of syringes, barely able to complete all of their assigned tasks.
|

FDA Establishment Inspection Report, June 5, 2005 |
|
|
Aug. 11, 2005
The FDA sends AM2PAT a warning letter, noting nine "significant violations" and asking the company to fix them.
|

FDA Warning Letter to AM2PAT, Aug 11, 2005 |
|
|
Sept. 14, 2005
AM2PATsends a response to the FDA describing changes it made in response to shortcomings identified in the August warning letter. The company sends a second response in October.
|
||
|
Nov. 15, 2005
|
||
|
Jan. 11, 2006
AM2PAT begins shipping syringes that later form the basis for criminal charges of mail fraud against Dushyant Patel. The allegations say Patel shipped syringes labeled "sterile" that had not undergone sterility testing.
|
||
|
Jan. 17, 2006
An FDA inspector spends four days at the AM2PAT plant. The inspection report notes that most deficiencies are fixed. Problems remain with validation of a filling machine and records discrepancies.
|

FDA Establishment Inspection Report, Jan. 17, 2006 |
|
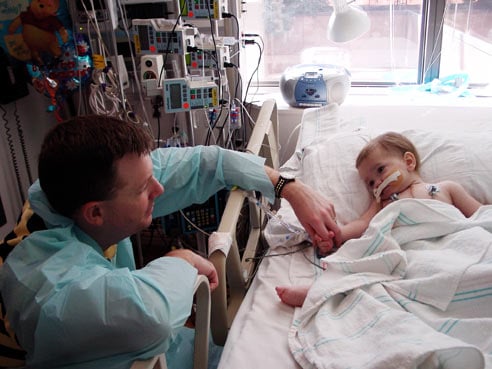
Leslie Fullerton and his daughter Natalie, who underwent a double-lung transplant operation. Fullerton family, photo dated Oct. 20, 2006 |
Oct. 16, 2006
Natalie Beth Fullerton, 14 months, survives a double-lung transplant. Her father will later use an AM2PAT syringe to clear a tube implanted near the little girl's heart. The syringe came from a lot of AM2PAT syringes that was later found to be contaminated with bacteria. Related story.
|
|
|
Mar. 19, 2007
|
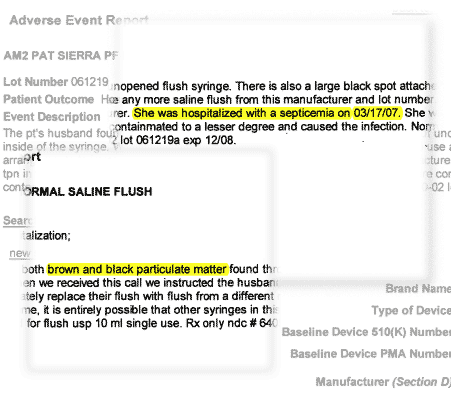
Adverse Event Report to FDA, Mar. 19, 2007 |
Mar. 17, 2007
A patient is hospitalized with septicemia, a blood infection, after using saline-filled syringes manufactured by AM2PAT. Contamination in the syringes is the suspected cause.
|
|
May 2007
AM2PAT moves its manufacturing facility from Raleigh, N.C., to Angier, N.C. An employee later tells criminal investigators that by this point, all lots of syringes are being shipped before samples are tested for sterility. The company does not notify the FDA of the move.
|

Photo of the Angier plant from the FDA Establishment Inspection Report, Dec. 13, 2007 |
|
|
May 23, 2007
AM2PAT begins making syringes that will elicit complaints of "white wispy matter" in the solution. The firm's customer recalls the syringes on July 30.
|
||
|
June 6, 2007
The FDA receives another complaint about white powder in a heparin-filled syringe manufactured by AM2PAT.
|
||
|
June 20, 2007
An FDA worker logs a complaint from an AM2PAT worker who says the correct temperature is not maintained in the "clean" room. The employee also says management ignored employee complaints about this. The FDA logs the complaint as "surveillance information" for its next inspection.
|
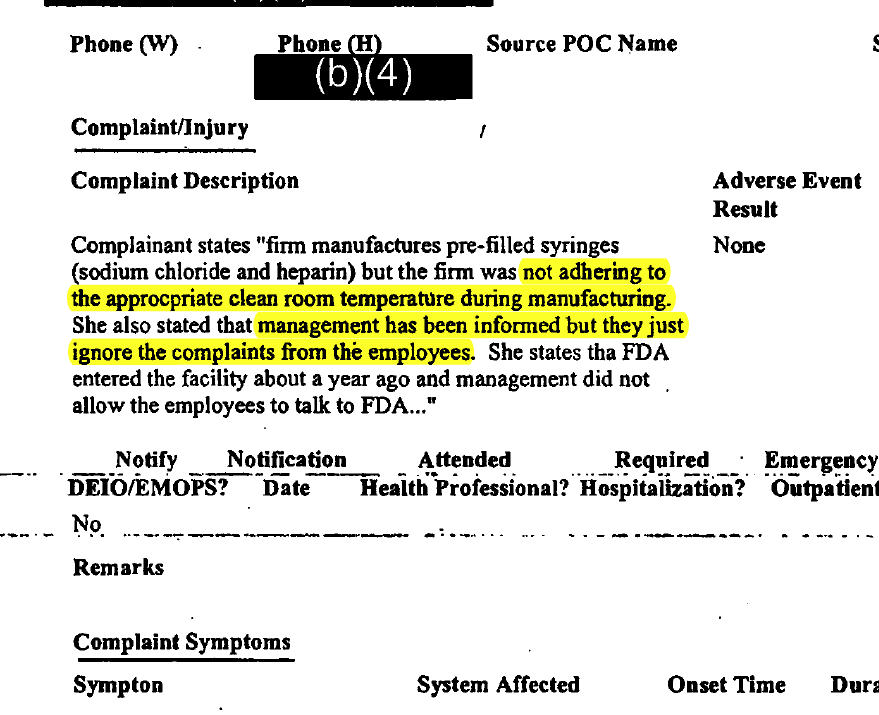
Consumer Complaint/Injury Report to the FDA, June 20, 2007 |
|
|
June 26, 2007
An FDA inspector goes to AM2PAT's Raleigh facility to investigate a report of contaminated syringes but finds the building shuttered.
|
||
|
July 30, 2007
B. Braun Medical, a Pennsylvania-based distributor of AM2PAT syringes, notifies its customers about a recall of 1.3 million saline-filled syringes. The recall follows reports of particles in the syringes. The FDA doesn't receive notice of B. Braun's action until a week later, as FDA policies do not set a deadline for companies to report a recall.
|

|
|
|
Aug. 1, 2007
An Atlanta-based inspector, unaware of the recall, goes to the firm's new location in Angier, N.C. She is aware of reports of red, black and brown particles in syringes, and notes that managers have a plan to combat rust contamination. She walks through the plant, her report says, but it does not indicate that she took any samples of the syringe fluid or that she checked to be sure the plant's machines were calibrated after the May 2007 move.
|
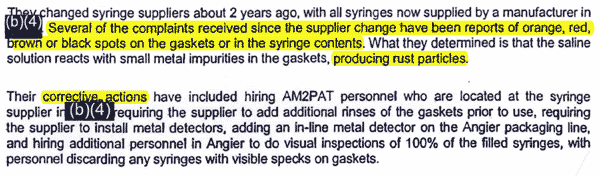
FDA Establishment Inspection Report, Aug. 1, 2007 |
|
|
Aug. 7, 2007
The FDA's Philadelphia office receives a notification in the mail about the B. Braun recall.
|
||
|
Sept. 14, 2007
B. Braun and the FDA publicly announce the recall of 1.3 million syringes, noting that reports of silicone particles floating in the fluid prompted the action. A news release says there were no patient injuries, but the long-term use of the syringes could potentially cause brain damage or a deadly embolism.
Despite FDA guidelines that call for an inspection to root out the cause of a significant recall, the agency does not initiate one, a spokeswoman said. |

FDA Press Release, Sept. 14, 2007 |
|
|
October 2007
AM2PAT ships one lot of syringes that are later linked to four deaths and 162 infections, according to the Centers for Disease Control.
|

Leslie Fullerton, 37, holds two syringes from the same lot of syringes as those that are believed to have infected his daughter, Natalie Beth Fullerton. Chris Walker/Chicago Tribune, May 11, 2009 |
|

Kyle Pacheco greets his mother Kathleen in San Francisco. Family photo, Oct. 2007 |
Oct. 21, 2007
Kyle Pacheco, 19, of Lake Worth, Fla., greets his mother at the finish line of a marathon in San Francisco to raise money for leukemia patients. At this point, he has been cancer-free for 20 months. He will later use a syringe from the tainted AM2PAT lot and fall into a coma, his lawyer Robert Boyers said.
|
|
|
Nov. 13, 2007
AM2PAT receives a complaint saying patients in a hospital are developing blood poisoning. Company President Dushyant Patel did not forward the complaint to the FDA, records show. He asked distributors to cease distributing one lot of syringes, according to his statement to FDA inspectors.
|
||
|
Nov. 28, 2007
Katie Abrams, a leukemia patient from the Chicago area, begins using an AM2PAT syringe. By Dec. 2, Abrams starts shaking uncontrollably, vomiting and develops a fever of 105.5 degrees, according to her personal injury lawsuit in Cook County Circuit Court. She is rushed to the emergency room, where she was diagnosed with a bacterial infection and hospitalized for about 10 days, a lawsuit filed in Cook County says.
|
||
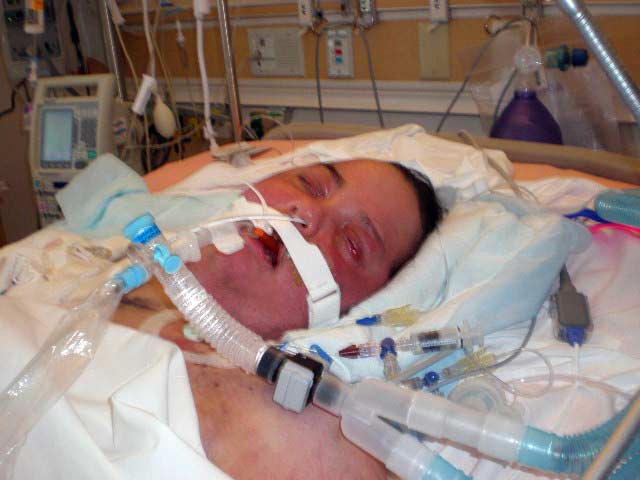
Kyle Pacheco undergoing treatment in Feb. 2008 from an infection his attorney alleges came from using tainted heparin in Nov. 2007. Family photo, Feb. 2008 |
Nov. 29, 2007
Kyle Pacheco, the Florida teen, is rushed to the hospital after the tainted fluid from a lot of tainted syringes is injected into a catheter in his right arm and delivered to vein above his heart, according to his civil lawsuit filed in south Florida federal court. The serratia bacteria from the syringe puts him in a coma for a month and renders him brain damaged and barely able to walk, his attorney said.
|
|

Leslie and Natalie Fullerton. Fullerton family photo |
Dec. 1, 2007
Leslie Fullerton, of Conroe, Texas, gives heparin from a lot of tainted syringes to his daughter, Natalie Beth Fullerton, 2. Just over a week later, the toddler is admitted to Texas Children's Hospital with a fever, chills and bloodstream infection. She only leaves the hospital at Christmas to be with her family.
|
|
|
Dec. 3, 2007
A special committee under the FDA's science advisory committeereleases a report identifying multiple failings in within the FDA's science, communication and technology systems. The shortcomings and lack of resources are so great that "American lives are at risk," the report says.
|

Jim Tallian at his parents' home in Palatine, Ill. He suffered an infection while being treated for leukemia. Chris Walker/Chicago Tribune, May 14, 2009 |
Dec. 3, 2007
Jim Tallian, of the Chicago suburbs, suddenly becomes seriously ill with a high fever and infection-related symptoms, according to a lawsuit filed in Cook County Circuit Court. He was hospitalized for several days. Unaware that his syringes might be contaminated, he used another syringe the day after he left the hospital, fell ill and was readmitted for several days, according to his lawsuit filed in Cook County.
|
|
Dec. 10, 2007
Doris Wickman, a physician in the Chicago suburbs, uses an AM2PAT syringe to flush out an intravenous line used for chemotherapy, Chicago attorney Patrick Hurley said. Hours later, the hospital that delivered the syringe to her warns her not to use it. It is too late – Wickman feels so feverish and ill that she checks into another hospital until Christmas Day, her attorney said. In the hospital, she is diagnosed with a bacterial infection. As a result of being treated with antibiotics for the infection, she suffers partial hearing loss in her right ear, for which there is no treatment or relief with a hearing aid, her lawyer said.
|
||
|
Dec. 11, 2007
State health departments and Centers for Disease Control tie one lot of AM2PAT's syringes to clusters of illness in Texas and Illinois and notify the FDA of the reports.
|
Dec. 12, 2007
Chicago-area resident Sheila Aleckson, who was being treated for short bowel syndrome, was getting better – gaining weight and regaining her strength – when she uses an AM2PAT heparin-filled syringe, according to her attorney. She suddenly becomes seriously ill. She is diagnosed with sepsis, a life-threatening blood infection.
|
|
|
Dec. 13, 2007
Three FDA officials begin a 14-day inspection at AM2PAT, discovering "official" and "unofficial" sets of product history records. Company President Dushyant Patel alleges that someone is "sabotaging" the records. Inspectors find brown water coming out of the faucets at the plant and snap photos of fans held together with duct tape in a room that should be sterile.
Dec. 20, 2007
AM2PAT, via a news release sent by the FDA, recalls a lot of 74,000 syringes that was determined by the Centers for Disease Control on Dec. 11 to have infected multiple patients.
|

FDA Establishment Inspection Report, Dec. 13, 2007 
Photo from FDA Establishment Inspection Report, Dec. 13, 2007 
FDA Establishment Inspection Report, Dec. 13, 2007 |
|
|
Jan. 16, 2008
AM2PAT officially closes.
|

AM2PAT workers inspecting syringes. Photo from FDA Establishment Inspection Report, Dec. 13, 2007 |
|
|
Jan. 18, 2008
|
||
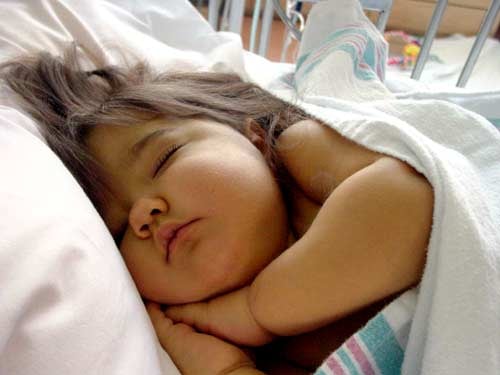
Natalie Fullerton, several days before her death. Fullerton family photo |
March 12, 2008
Natalie Beth Fullerton, 2, dies months after using an AM2PAT syringe. A Cook County, Ill., lawsuit filed by her family claims a contaminated syringe from AM2PAT compromised her health and triggered complications that led to her death.
|
|
|
April 2008
Federal prosecutors in North Carolina file a complaint against AM2PAT managers Ravindra Sharma of Richmond, Va. and Aniruddha Patel of Carpentersville, Ill., alleging fraud and introducing adulterated drugs into the market.
|
||
|
Aug. 1, 2008
A criminal complaint is entered against AM2PAT President Dushyant Patel. Authorities believe he has fled to India.
|
||
|
Aug. 21, 2008
AM2PAT's insurer goes to federal court in Chicago, asking a judge's direction on how to split up AM2PAT's $2 million liability insurance among multiple victims and attorneys.
|
||
|
Feb. 19, 2009
A grand jury in the Eastern District of North Carolina issues a superseding indictment against Dushyant Patel alleging mail fraud, conspiracy and introducing adulterated and misbranded medical devices into commerce. If convicted on all counts, Patel could be sentenced to 95 years in prison.
|
||
|
Feb. 23, 2009
Ravindra Sharma and Aniruddha Patel are each sentenced to serve 4 ½ years in prison in federal court in the eastern district of North Carolina. Both had accepted a guilty plea on one count of conspiracy to introduce a misbranded device into commerce.
Feb. 26, 2009
President Barack Obamaseeks the largest budget increase ever for the FDA. Nearly half of the additional $511 million is expected to go toward food-safety inspections. About $166 million is expected to support practices leading to safer food and drugs.
|

Aniruddha Patel, left, plant manager for the medical company AM2PAT, walks to the federal courthouse in Raleigh, N.C., with his lawyer Dan Boyce, right. AP Photo/The News & Observer/Shawn Rocco, Feb. 23, 2009 |
|
|
April 2009
Federal prosecutors submit paperwork to Interpol to secure the detention of AM2PAT President Dushyant Patel.
|



